Researchers have developed a novel approach that combines magnetic fields and electrocatalysis to improve fuel production efficiency. By surrounding the catalysts with magnetic fields, they observed enhanced movement of reactants and products at the catalyst surface, leading to a more than 50% boost in activity for the oxygen reduction reaction. This breakthrough has the potential to revolutionize energy conversion technologies and advance sustainable fuel production.
Enhancing Fuel Production Efficiency with Magnetic Fields and Electrocatalysis
In the pursuit of sustainable energy sources, researchers are continuously exploring new avenues to improve fuel production processes. Electrocatalysis, which involves using catalysts to speed up electrochemical reactions, plays a crucial role in converting chemical energy into electrical energy and vice versa. It is widely used in green-energy technologies such as fuel cells and electrolyzers.
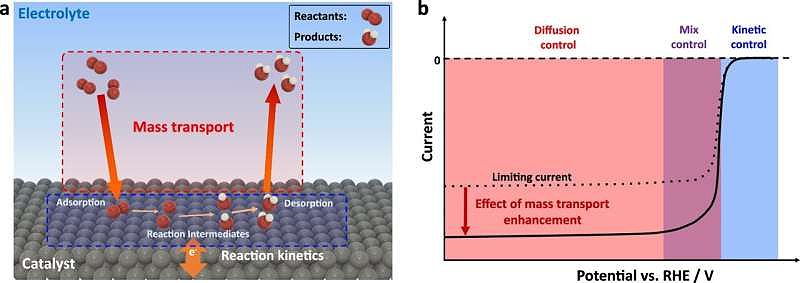
However, traditional electrocatalysis methods often struggle to maximize the transport of reactants to the catalyst's surface, resulting in lower overall efficiency. This limitation hampers our progress towards clean energy solutions. To address this challenge, a team of scientists led by Magalí Lingenfelder at EPFL has developed a novel approach that combines magnetic fields and electrocatalysis to enhance fuel production efficiency.
The Intersection of Magnetic Fields and Electrocatalysis
The study, published in Nature Communications, focused on the intersection of magnetic fields and electrocatalysis. By surrounding the catalysts with magnetic fields, the researchers observed the creation of Lorentz forces. These forces, exerted by magnetic fields on moving electric charges, induced whirling motions that enhanced the movement of reactants and products at the catalyst surface. This improved the reaction's consistency and speed, overcoming limitations posed by reactant scarcity, particularly in reactions critical for fuel cells, such as the oxygen reduction reaction (ORR).
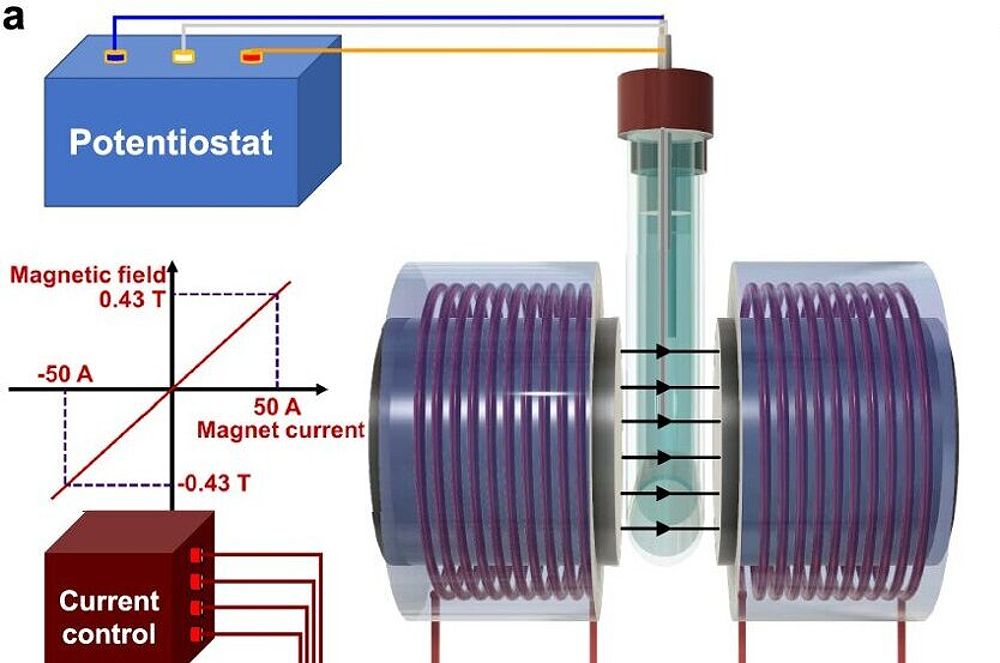
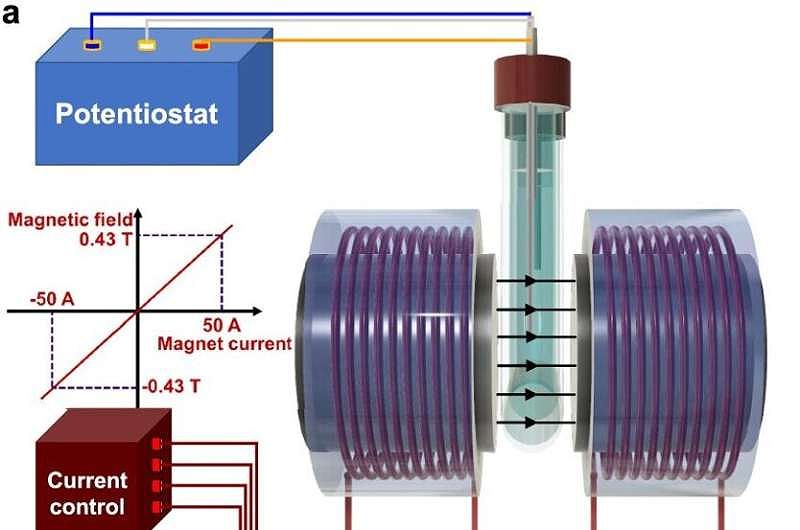
To observe the movement of ions in real time under a magnetic field, the researchers developed an advanced magneto-electrochemical setup. They collaborated with spintronics expert Professor Jean-Philippe Ansermet, who had previously studied spin effects in electrochemistry, to adapt an electromagnet for measuring magnetic field effects on key electrocatalytic reactions for green energy. Using a creative approach developed by the first authors of the study, Priscila and Yunchang, the team tracked how ions moved in the electrolyte under a magnetic field, providing valuable insights on how to apply magnetic fields to enhance electrocatalysis in a reproducible manner.
Boosting Efficiency through Magnetic Fields
By applying magnetic fields to non-magnetic electrodes and monitoring reactions, the scientists were able to decouple different effects and observe how magnetic forces stirred and enhanced the movement of reactants around the catalyst. This process, akin to creating miniature whirlpools, significantly improved the efficiency of reactions crucial for green hydrogen production. The study demonstrated a more than 50% boost in activity for the oxygen reduction reaction induced by magnetic fields on non-magnetic interfaces, representing a substantial jump in efficiency.
Furthermore, the study resolved several fundamental controversies in the field by elucidating the mechanisms and conditions required for magnetic fields to enhance different electrocatalytic reactions involving gas products or reactants like hydrogen and oxygen. This research paves the way for using magnetic fields to improve the efficiency of electrocatalysis, offering a promising avenue for advancing sustainable fuel production. It has the potential to revolutionize energy conversion technologies, increase the adoption of fuel cells in hydrogen vehicles, and boost the production of hydrogen as a clean energy source, thereby mitigating the impact of our energy consumption on climate change.