Biosense Webster has obtained CE mark approval for its VARIPULSE Platform, a pulsed field ablation system designed to treat drug refractory recurrent paroxysmal atrial fibrillation. The platform offers an intuitive workflow with real-time visualization, showcasing safety and efficacy in clinical trials.
Biosense Webster Receives CE Mark Approval for VARIPULSE Platform for Treating Atrial Fibrillation
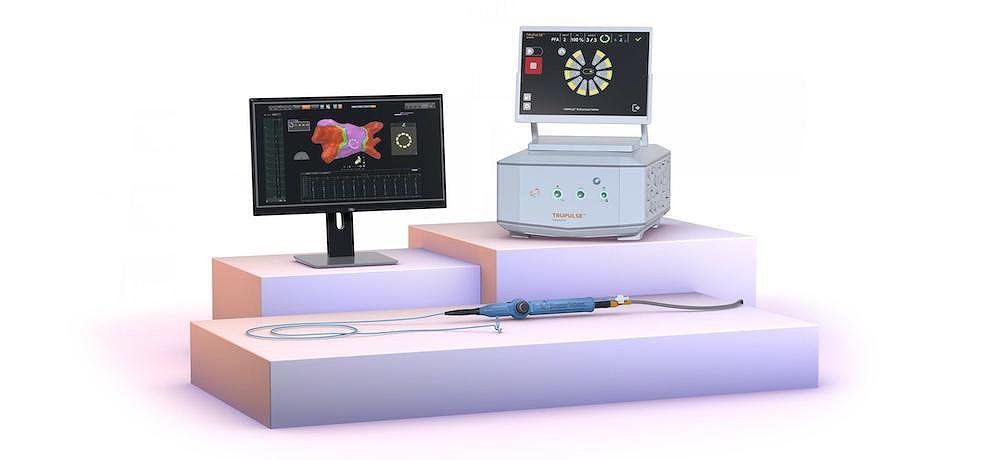
Biosense Webster, a specialist in treating cardiac arrhythmia, has obtained CE mark approval for its VARIPULSE Platform. This platform is designed for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA). The VARIPULSE Platform consists of the VARIPULSE Catheter, the TRUPULSE Generator, and the CARTO 3 System.
The VARIPULSE Platform is the only CARTO integrated PFA system, providing an intuitive and reproducible workflow with real-time visualization and feedback mechanisms. It has undergone rigorous testing in the inspIRE trial, which involved 186 patients in Canada and Europe. Updated one-year follow-up data was recently presented at the AF Symposium in Boston, showcasing the platform's safety and efficacy.
Superior Treatment for Atrial Fibrillation
The study revealed that among participants who received optimal PFA applications, 80% achieved freedom from recurrence with no primary adverse events. Additionally, the primary effectiveness endpoint (PEE) of acute pulmonary vein isolation and 12-month freedom from atrial arrhythmia recurrence (AF, Atrial Tachycardia, or Atrial Flutter) was 75.6%. The VARIPULSE Platform also demonstrated a low fluoroscopy time of 7.8 minutes, thanks in part to its integration with the CARTO 3 System. Importantly, there were no reported primary adverse events.
Tom De Potter, associate director at the Cardiovascular Center of OLV Hospital Aalst in Belgium, commented on the significance of the VARIPULSE Platform's CE mark approval. He emphasized that it represents a significant advancement in catheter ablation technology, allowing electrophysiologists in Europe to offer patients pulsed field ablation treatment with real-time integrated 3D mapping. The platform's integration with the CARTO 3 System streamlines the workflow and reduces fluoroscopy time.
Pulsed Field Ablation: A New Approach to Treating Atrial Fibrillation
Catheter ablation is a minimally invasive procedure used to treat heart rhythm disorders, including AF. The VARIPULSE Platform introduces a new approach to AF treatment with pulsed field ablation, which utilizes a controlled electric field to selectively ablate cardiac tissue causing the irregular heartbeat. This process, called irreversible electroporation (IRE), minimizes thermal energy and reduces the risk of damage to surrounding tissues.
Jasmina Brooks, president of Biosense Webster, expressed the company's commitment to pushing the boundaries of science and technology in cardiac ablation. The CE mark approval of the VARIPULSE Platform demonstrates this commitment, offering healthcare professionals the potential to improve outcomes for people living with atrial fibrillation. Pulsed field ablation has the potential to offer safer, more consistent, and efficient workflows, and the VARIPULSE Platform provides physicians with a simple and reproducible PFA workflow with real-time 3D visualization.